Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The structural formula is
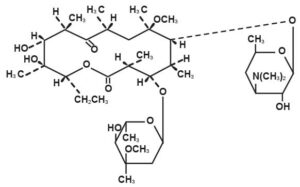
Clarithromycin is a white to off-white crystalline powder. It is soluble in acetone, slightly soluble in methanol, ethanol, and acetonitrile, and practically insoluble in water.
MACLACIN is available as 250mg and 500mg film coated tablets and granules 125mg/5ml and 250mg/5ml for oral suspensions.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Clarithromycin is rapidly absorbed from the gastrointestinal tract after oral administration. The absolute bioavailability of 250mg clarithromycin tablets was approximately 50%. For a single 500mg dose of clarithromycin, food slightly delays the onset of clarithromycin absorption, increasing the peak time from approximately 2 to 2.5 hours. Food also increases the clarithromycin peak plasma concentration by about 24%, but does not affect the extent of clarithromycin bioavailability.
Food does not affect the onset of formation of the antimicrobially active metabolite, 14-OH clarithromycin or its peak plasma concentration but does slightly decrease the extent of metabolite formation, indicated by an 11% decrease in area under the plasma concentration-time curve (AUC). Therefore, Maclacin tablets may be given without regard to food.
In nonfasting healthy human subjects (males and females), peak plasma concentrations were attained within 2 to 3 hours after oral dosing. Steady-state peak plasma clarithromycin concentrations were attained within 3 days and were approximately 1 to 2 μg/mL with a 250mg dose administered every 12 hours and 3 to 4 μg/mL with a 500mg dose administered every 8 to 12 hours. The elimination half-life of clarithromycin was about 3 to 4 hours with 250mg administered every 12 hours but increased to 5 to 7 hours with 500mg administered every 8 to 12 hours. The nonlinearity of clarithromycin pharmacokinetics is slight at the recommended doses of 250mg and 500mg administered every 8 to 12 hours. With a 250mg every 12 hours dosing, the principal metabolite, 14-OH clarithromycin, attains a peak steady-state concentration of about 0.6 μg/mL and has an elimination half-life of 5 to 6 hours. With a 500mg every 8 to 12 hours dosing, the peak steady-state concentration of 14-OH clarithromycin is slightly higher (up to 1 μg/mL), and its elimination half-life is about 7 to 9 hours. With any of these dosing regimens, the steady-state concentration of this metabolite is generally attained within 3 to 4 days.
After a 250mg tablet every 12 hours, approximately 20% of the dose is excreted in the urine as clarithromycin, while after a 500mg tablet every 12 hours, the urinary excretion of clarithromycin is somewhat greater, approximately 30%. In comparison, after an oral dose of 250mg (125mg/5mL) suspension every 12 hours, approximately 40% is excreted in urine as clarithromycin. The renal clearance of clarithromycin is, however, relatively independent of the dose size and approximates the normal glomerular filtration rate. The major metabolite found in urine is 14-OH clarithromycin, which accounts for an additional 10% to 15% of the dose with either a 250mg or a 500mg tablet administered every 12 hours.
Steady-state concentrations of clarithromycin and 14-OH clarithromycin observed following administration of 500mg doses of clarithromycin every 12 hours to adult patients with HIV infection were similar to those observed in healthy volunteers. In adult HIV-infected patients taking 500mg or 1000mg doses of clarithromycin every 12 hours, steady-state clarithromycin Cmax values ranged from 2 to 4μg/mL and 5 to 10μg/mL, respectively. The steady-state concentrations of clarithromycin in subjects with impaired hepatic function did not differ from those in normal subjects; however, the 14-OH clarithromycin concentrations were lower in the hepatically impaired subjects. The decreased formation of 14-OH clarithromycin was at least partially offset by an increase in renal clearance of clarithromycin in the subjects with impaired hepatic function when compared to healthy subjects.
The pharmacokinetics of clarithromycin was also altered in subjects with impaired renal function. Clarithromycin and the 14-OH clarithromycin metabolite distribute readily into body tissues and fluids. There are no data available on cerebrospinal fluid penetration. Because of high intracellular concentrations, tissue concentrations are higher than serum concentrations. Examples of tissue and serum concentrations are presented below.
| CONCENTRATION (After 250mg q12h) | ||
| Tissue Type | Tissue (μg/g) | Serum (μg/mL) |
| Tonsil | 1.6 | 0.8 |
| Lung | 8.8 | 1.7 |
When 250mg doses of clarithromycin as Maclacin suspension were administered to fasting healthy adult subjects, peak plasma concentrations were attained around 3 hours after dosing.
Steady-state peak plasma concentrations were attained in 2 to 3 days and were approximately 2μg/mL for clarithromycin and 0.7μg/mL for 14-OH clarithromycin when 250mg doses of the clarithromycin suspension were administered every 12 hours. Elimination half-life of clarithromycin (3 to 4 hours) and that of 14OH clarithromycin (5 to 7 hours) were similar to those observed at steady state following administration of equivalent doses of Maclacin tablets.
For adult patients, the bioavailability of 10mL of the 125mg/5mL suspension or 10mL of the 250mg/5mL suspension is similar to a 250mg or 500mg tablet, respectively.
In children requiring antibiotic therapy, administration of 7.5mg/kg q12h doses of clarithromycin as the suspension generally resulted in steady-state peak plasma concentrations of 3 to 7μg/mL for clarithromycin and 1 to 2μg/mL for 14-OH clarithromycin.
In HIV-infected children taking 15mg/kg every 12 hours, steady-state clarithromycin peak concentrations generally ranged from 6 to 15μg/mL.
Clarithromycin penetrates into the middle ear fluid of children with secretory otitis media.












Reviews
Clear filtersThere are no reviews yet.